
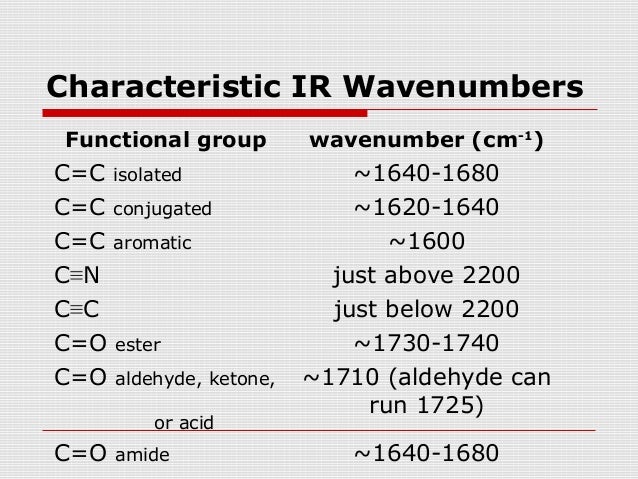
"Vibrational and Electronic Energy Levels of Polyatomic Transient Molecules. Complete the following IR spectroscopy table by assigning the correct frequency, placed in random order and position, to each functional. ^ NSRDS-NBS: National Standard Reference Data Series, National Bureau of Standards (PDF).Due to historical reasons however, we typically discuss IR light in spectroscopy in terms of wavenumbers rather than wavelengths. It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms. Infrared light is part of the electromagnetic spectrum between visible light and microwaves, with wavelengths ranging from 780 nm to 1 mm. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Applications in Coordination, Organometallic, and Bioinorganic Chemistry. Infrared spectroscopy ( IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. If you were to look up - on a 'table of characteristics of IR absorption' - you would see that a signal appearing at approximately 2100-2260 cm-1 depicts a. Infrared and Raman Spectroscopy Principles and Spectral Interpretation. Infrared and Raman Characteristic Group Frequencies: Tables and Charts. Two bands (distinct from ketones, which do not possess a C─O bond) The melanin Raman spectrum is dominated by two intense and broad peaks at about 15 cm 1, which can be interpreted as originating from the in-plane stretching of the aromatic rings and the linear stretching of the C-C bonds within the rings, along with some contributions from the C-H vibrations in the methyl and methylene groups. Influenced by conjugation and ring size (as with ketones) Influenced by conjugation (as with ketones) Tables of vibrational transitions of stable and transient molecules are also available. IR spectroscopy is useful when it comes to analysis of inorganic compounds (such as metal complexes or fluoromanganates) as well. This research aims to establish an effective method to determine the quantification of chlorophyll and pheophytin in green tea, based on Fourier transform infrared (FTIR) spectroscopy. The absorptions in this range do not apply only to bonds in organic molecules. Go to: Abstract The chlorophyll, pheophytin, and their proportions are critical factors to evaluate the sensory quality of green tea. In physical and analytical chemistry, infrared spectroscopy (IR spectroscopy) is a technique used to identify chemical compounds based on the way infrared radiation is absorbed by the compound.

Further information: Infrared spectroscopyĪn infrared spectroscopy correlation table (or table of infrared absorption frequencies) is a list of absorption peaks and frequencies, typically reported in wavenumber, for common types of molecular bonds and functional groups.


 0 kommentar(er)
0 kommentar(er)
